
Ultrasensitive
Detects biomarker even highly diluted . Detects as low as 7 aberrant molecules per 1.000 normal molecules.

A Reliable and Non-invasive Urine test, based on a Patented method that assesses a specific set of FGFR3/TERTp biomarker hotspot mutations which are 100% Sensitivity and 97.3% Specificity for detecting bladder cancer (BC), independently of tumor stage and of grade, Outperforming cytology in detecting recurrence of non-muscle invasive BC and allowing a reduction in cystoscopies, more convenient for the patients and at Lower Cost.
Test was validated in a Multicentric study and at Reference Urology Center: Radboud UMC (Netherlands)
| Year 1 / Year 2 | Year 3 / Year 4 | Year 5 |
|---|---|---|
| 4 cystocopies/year | 2 cystocopies/year | 1 cystocopies/year |

Detects biomarker even highly diluted . Detects as low as 7 aberrant molecules per 1.000 normal molecules.
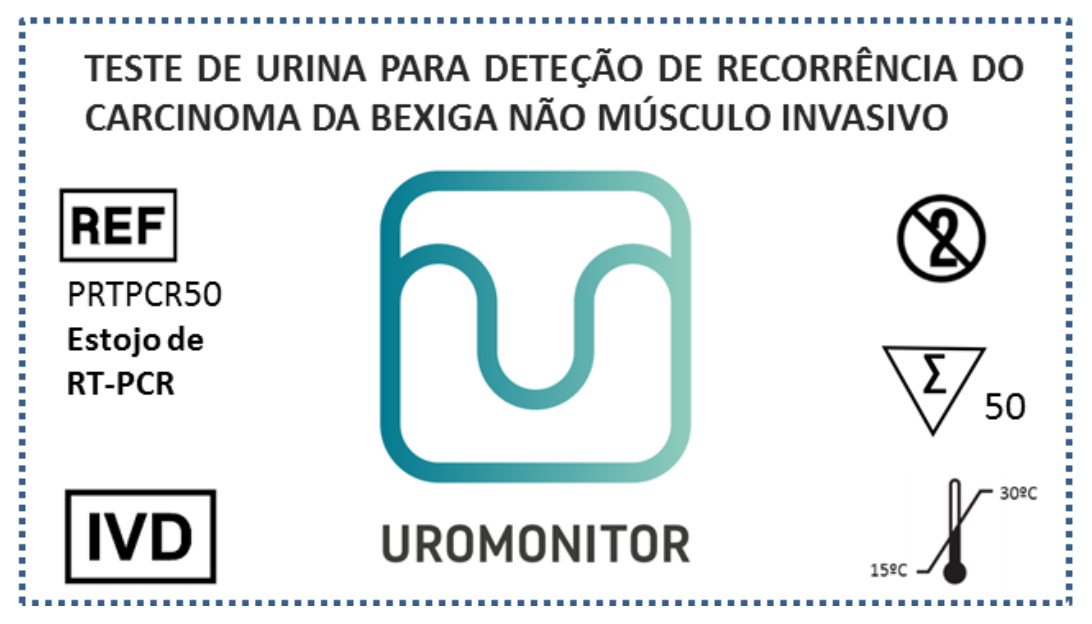
Urine samples, collected through a filter that can be sent to a central Lab at room temperature.

Patented by an International-scope PCT Application.
National Offices validation in US; CN; BR; EU; CN; IN; RS; AU; JP.
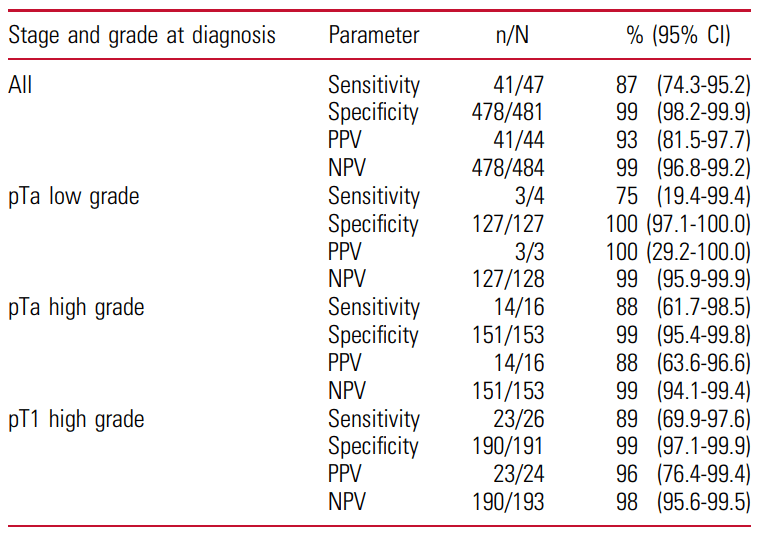

The objective of the present study was to prospectively evaluate FGFR mutation detetion in matched urine and tissue samples from patients suspicious of bladder cancer and undergoing first TURB within the framework of the BRIDGister RealWorld Experience trial. Results: The pilote cohort of the BRIDGister trial consisted of 47 patients (median age: 77, male 65% vs… Read more »

The Uromonitor test was recently validated by the scientific community in a international recognized peer-reviewed journal (Frontiers in Genetics) in a multicentric study comprising more than 300 human samples. Uromonitor presented high sensitivity (74%) and specificity (93%) in the detection of recurrence in patients under Follow-up for Non-Muscle Invasive Bladder Cancer. The details of… Read more »

Uromonitor test was recognized and considered by the scientific community as an excellent alternative for the non-invasive follow-up of patients previously diagnosed with Non-Muscle Invasive Bladder Cancer in a scientific article that was issue cover in an recognized international journal (Diagnostics). Bladder cancer (BC) ranks as the sixth most prevalent cancer in the world, with… Read more »

Recently, investigators described in a scientific publications in a recognized international journal (International Journal of Molecular Sciences), the future possibility of the use of an genetic alterations in TERT gene, included in the Uromonitor test, to be used as a therapy response predictive biomarker in BCG treatment. The details of this study can be found… Read more »

Table 3. Comparing Uromonitor-V2 Test Characteristics with urine cytology.

Figure 1. comparing ROC curves of urine cytology (red) with Uromonitor-V2 (black) for patients with a historic of NMIBC.

Table 2. Test performances in enrolled patients (n = 97), per subgroup.

Uromonitor Performance (Histology Confirmed Recurrences)




(Distributors in 15 countries)

BsC, MBA









The test is currently performed as a service at Uromonitor, hosted at the above mentioned institutions.
To access the service, urologists in hospitals and clinics should first have materials for urine collection and shipment.
Direct ordering the complete analysis kit to be performed in a laboratory or medical unit.
Recommended for professionals!
“For persons at risk or patients diagnosed with bladder cancer, as well as their urologist, who have the need of more convenient surveillance methods, Uromonitor is a completely non-invasive, urine-based, In vitro Diagnosis (IVD) kit that is able to detect bladder cancer with high sensitivity and specificity.
Uromonitor is easy and can deliver reliable results to reduce costs with follow-up”.