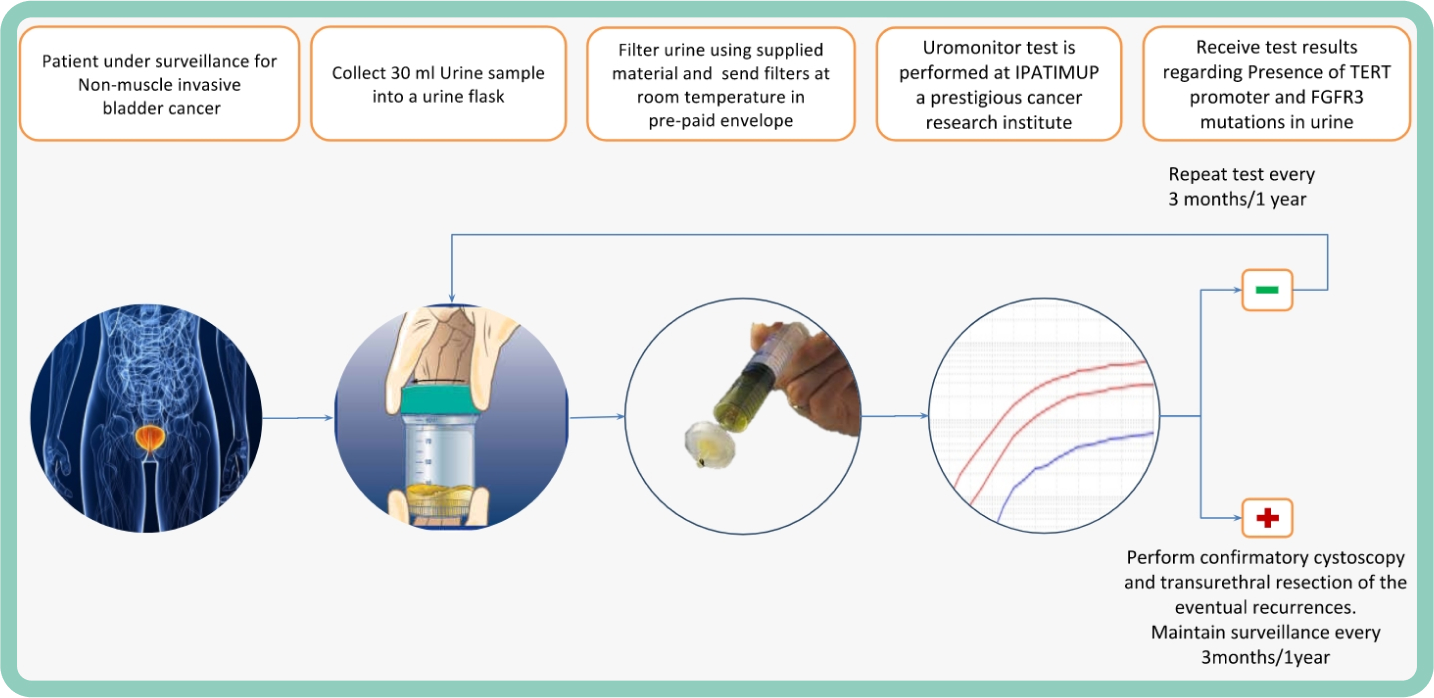
Prospective validation of urine based FGFR screening by Uromonitor within the real-world clinicopathological register trial BRIDGister
Monday, March 14th, 2022

The objective of the present study was to prospectively evaluate FGFR mutation detetion in matched urine and tissue samples from patients suspicious of bladder cancer and undergoing first TURB within the framework of the BRIDGister RealWorld Experience trial.
Results: The pilote cohort of the BRIDGister trial consisted of 47 patients (median age: 77, male 65% vs female 35%) of diverse clinical stages (benign lesions/no tumor 38%, pTa 23%, pT1 20%, pT2 19%) and WHO 1973 grade (G1 11%, G2 43%, G3 23%). Based on FFPE tissue testing using Therascreen FGFR IVD kit 10 out of 47 patients exhibited FGFR alterations (25%), while urine filtering for cellular components and subsequent PCR testing revealed 13 out of 40 matched urine sampels were FGFR positive (33%). Comparison with tissue testing as probable gold standard revealed 100% sensitivity, 90% specificity, 77% PPV, 100% NPV as well as high concordance (kappa 0,82, p < 0,0001). There were 3 patients being FGFR positive for Uromonitor from urine with no mutation found in the corresponding TUR biopsy. Conclusions: Filtering urine for cells and subsequent DNA extraction followed by PCR detection results in highly sensitive mutation testing being feasible with good concordance to matched tissue testing. Prospective testing validated the diagnostic accuracy of the Uromonitor FGFR test in a real world setting.
Please read the full article:
https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.6_suppl.471