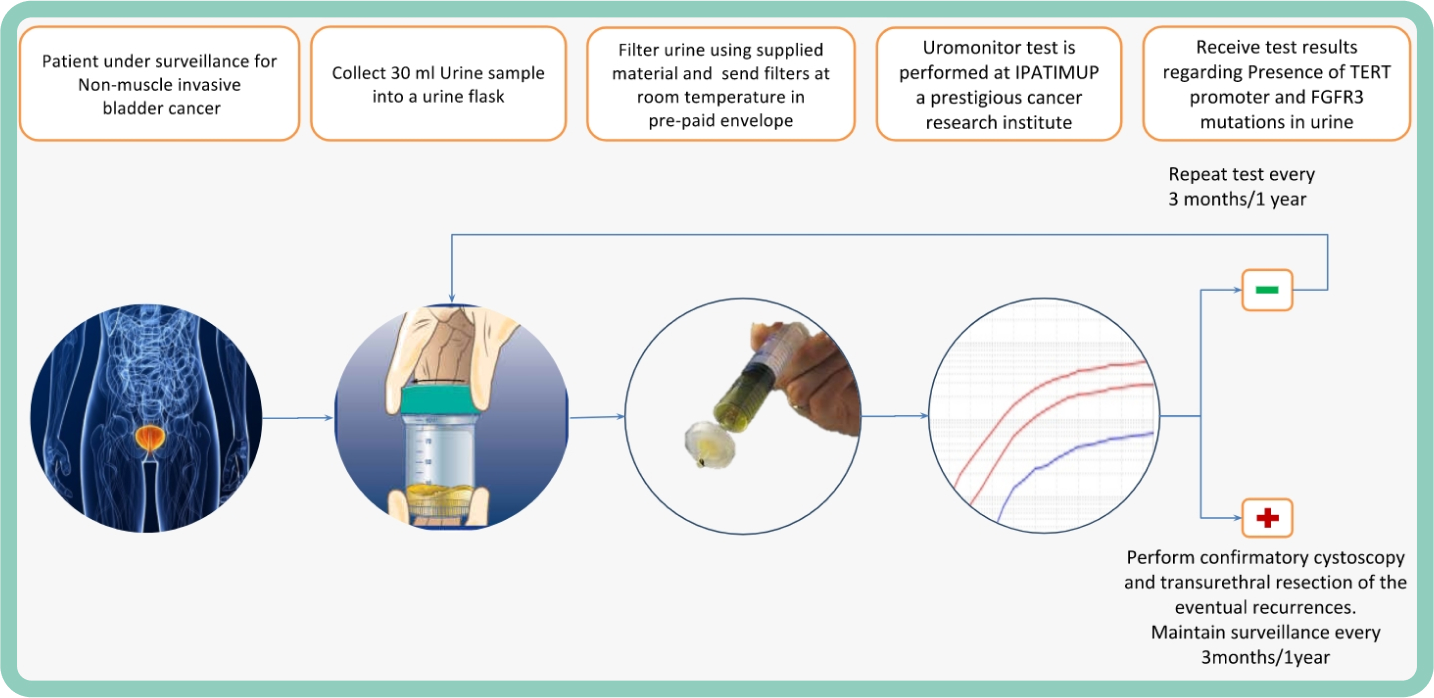
In times of covid19, cancer patients have a new way to avoid delaying diagnostic procedures and surveillance of bladder tumours.
Monday, December 27th, 2021
Concerned about the postponement of surveillance procedures and the increased risk of contamination in Hospitals, a team of researchers from Ipatimup and the Institute for Research and Innovation in Health (i3S) validated a new form of bladder cancer surveillance that, from now on, may be carried-out from home.
Bladder cancer mainly affects smoking men over 60 years of age, who also constitute a population with a higher risk of mortality from COVID19. Irrespective of the pandemic situation, these patients have to maintain a tight surveillance program as these tumours return with recurrences in 70% of patients. With the pandemic, many of these procedures have been postponed.
To face this problem, a new Kit is available that allows patinets to collect and filter their urine at home, without the need to go to the hospital. The Kit is part of the internationally patented test and registered with the trademark Uromonitor®, and represents a non-invasive and comfortable way of monitoring the appearance of bladder tumours in times of pandemic, since it is carried out from a urine sample obtained and filtered by the patient himself (self-collection).
“The filter containing biological material filtered from the urine is sent free of charge to the laboratory where an ultra-sensitive test is then carried out to detect trace amounts of genetic mutations associated with the early stages of bladder cancer in the urine”, explains Rui Batista, researcher that validated the samples taken at home.
The conventional method, based on cystoscopy with urinary cytology, has been postponed by patients, as it implies traveling to Hospitals, which express concerns of contagion with COVID19. However, surveillance of these tumours must be performed repeatedly, at intervals that can range from 3 months to 1 year, throughout the patient’s life. That is why patients like Mr. Caldeira have already chosen to take the test at home. As he explains: “Given my need to keep this type of cancer under surveillance, the possibility of postponing or going to the hospital did not make me feel safe”.
“The test itself is highly robust, with very high sensitivity and practically zero risk of false negatives, regardless of the stage”, as João Vinagre, a researcher involved in the clinical validation of the test, says.
As for Urologists, they are facing with high waiting lists and reduced teams due to the need to allocate resources to COVID19. For them, the possibility of using an auxiliary test with great sensitivity and diagnostic accuracy, especially in tumours of lower stages, avoiding hospital procedures and the risk of contamination of health personnel, represents a more than justified option in the context of the pandemic.
“For this reason, the home collection and filtration kit has the potential to be used as an alternative or at least as a complement to cystoscopy performed in a hospital setting, with the benefit of helping the urologist to reduce waiting lists and relieve patients of the trips they need to undergo, ”adds Ricardo Leão, Urologist.
“To carry out the test, you just need to request a Uromonitor test with self-collection and urine filtration kit, under medical prescription, and as part of the Urology follow-up carried out in clinics and hospitals, as explained by André Martins, responsible for logistics. After ordering, a urine collection and filtration kit that is compact enough to fit in the mailbox is sent to your home. After receiving the kit, just follow a simple set of instructions to send the laboratory, in a safe and freeway, a filter where the scaled biological material from the bladder is captured and preserved, as indicated by Fernando Vieira, responsible for operations.
This product represents an example of how, in this time of pandemic, scientific research can be mobilized to find innovative ways to respond to the problems raised by COVID19 to cancer patients, as explained by Paula Soares, the project’s scientific leader.