Diagnostics: Clinical Validation of a Urine Test (Uromonitor-V2®) for the Surveillance of Non-Muscle-Invasive Bladder Cancer Patients
Thursday, February 6th, 2025
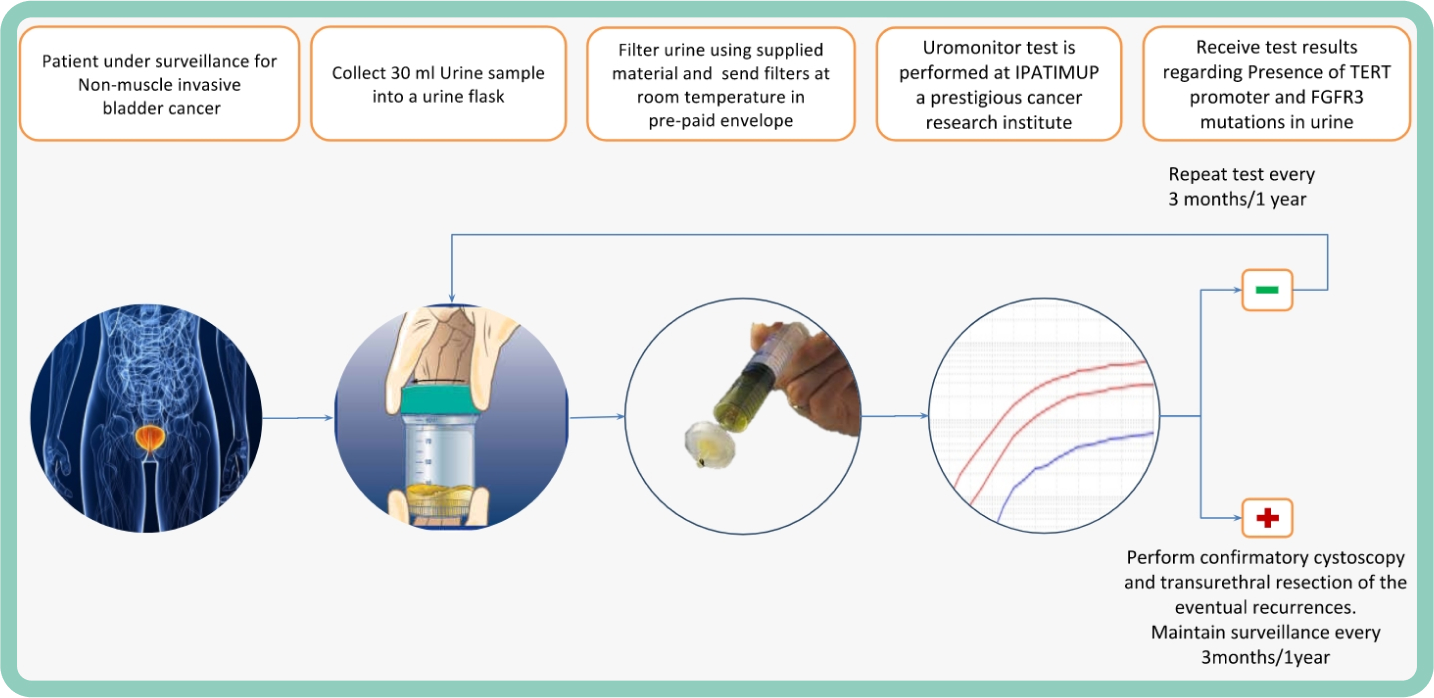
Uromonitor-V2®: A Breakthrough in NMIBC Surveillance
Bladder cancer (BCa) requires lifelong monitoring, but traditional methods like cystoscopy and urine cytology are invasive, costly, and often lack sensitivity.
Uromonitor-V2® is a non-invasive urine test that detects TERT, FGFR3, and KRAS mutations using real-time qPCR. Clinically validated, it outperforms urine cytology with 93.1% sensitivity and 85.4% specificity, ensuring high accuracy in detecting NMIBC recurrence. Its negative predictive value (NPV) of 95.3% provides confidence in ruling out recurrence.
With results in just six hours, Uromonitor-V2® offers a faster, more reliable, and cost-effective alternative to traditional surveillance methods.
At Uromonitor, we are committed to improving patient care by providing cutting-edge, accessible diagnostics. Our mission is to revolutionize NMIBC follow-up with a highly sensitive, non-invasive solution.
Read the Full Article