About
Wednesday, December 29th, 2021

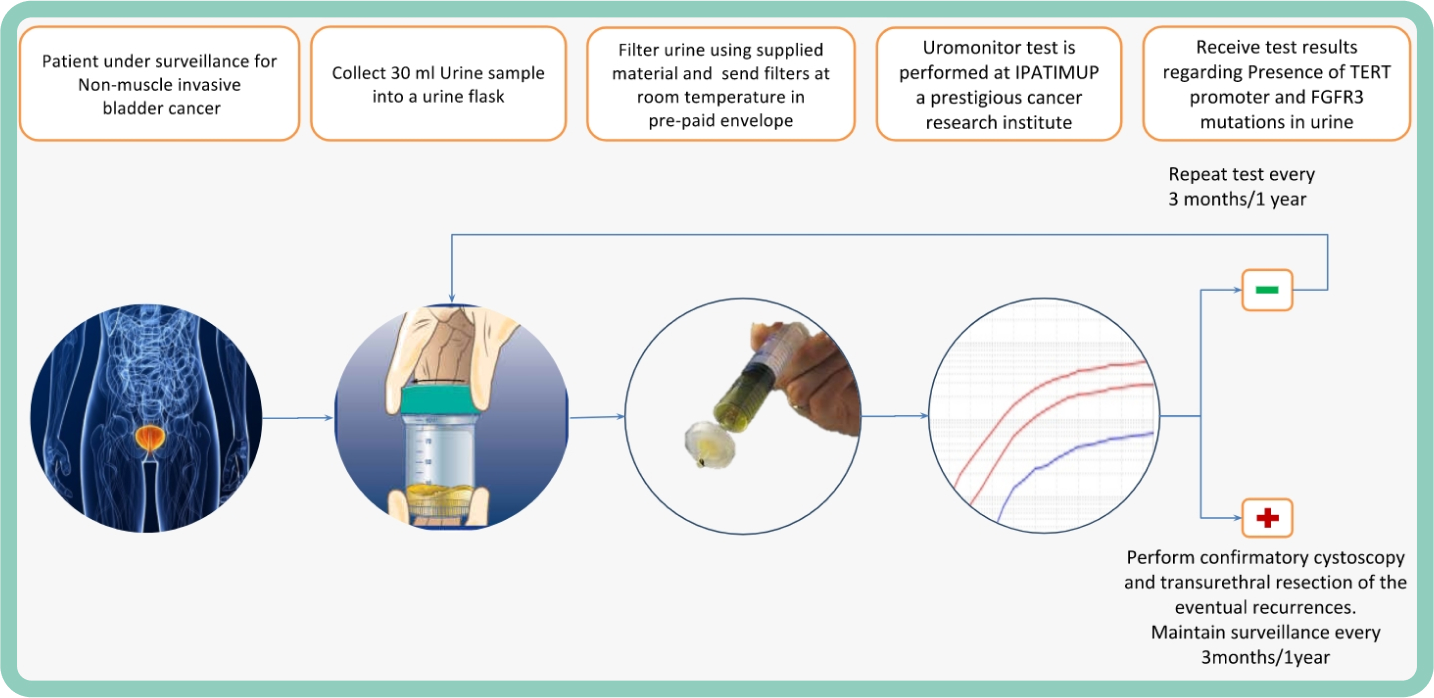
Uromonitor®_V2
Urine mutation test
Finally, a convenient way for patients and urologysts to manage the burden of bladder cancer survaillance.
A Reliable and Non-invasive Urine test, based on a Patented method that assesses a specific set of FGFR3/TERTp biomarker hotspot mutations which are 100% Sensitivity and 97.3% Specificity for detecting bladder cancer (BC), independently of tumor stage and of grade, Outperforming cytology in detecting recurrence of non-muscle invasive BC and allowing a reduction in cystoscopies, more convenient for the patients and at Lower Cost.
Test was validated in a Multicentric study and at Reference Urology Center: Radboud UMC (Netherlands)